Laser products entering the U.S.A. market are subject to FDA specific labeling rules that diverge from IEC / EN standards. However, Laser Notice 56 offers a streamlined path to compliance if applied correctly.
When manufacturing or importing laser products into the United States, compliance with the FDA’s Center for Devices and Radiological Health (CDRH) requirements under 21 CFR 1040.10 and 1040.11 is mandatory. These regulations differ from the international EN / IEC 60825-1 standard in several critical ways, particularly in how laser products must be labelled. Understanding these differences is essential for ensuring both market access and legal compliance.
To accommodate the challenges faced by manufacturers operating under multiple regulatory requirements, the FDA issued Laser Notice 56, formally acknowledging acceptance of IEC classification and labelling as an alternative to the CDRH system. As a result, manufacturers may now use the same labelling formats required under EN / IEC 60825-1 for products distributed in the U.S.A. market provided they comply with the conditions outlined in these notices.
Classification System Differences
While IEC 60825-1 uses Class 1, 1M, 2, 2M, 3R, 3B, and 4, the CDRH only recognizes Class I, II, IIIa, IIIb, and IV. This creates mapping discrepancies where, for example:
- IEC Class 1M maps to CDRH Class I but may need re-evaluation depending on measurement conditions.
- IEC Class 3R typically maps to CDRH Class IIIa.
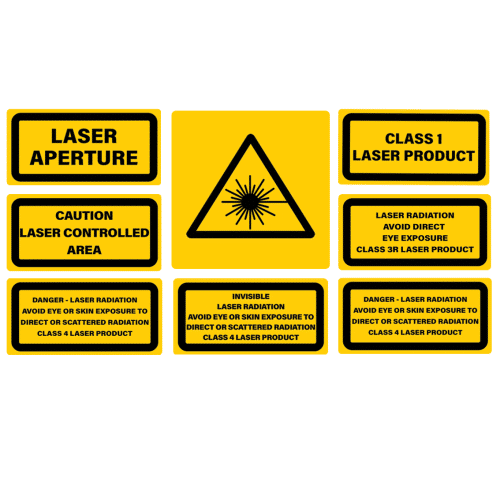
In this article, when Class 1, 2, 3, 4 is mentioned it refers to the laser classes in IEC 60825-1 and Class I, II, III, IV refer to the laser classes in 21 CFR 1040.10 and 1040.11.
Differences between IEC 60825-1 and 21 CFR Labelling Requirements
CLASSIFICATION LABEL, PRODUCT INFORMATION LABEL, & WARNING TRIANGLE
IEC/EN 60825-1 allows for a degree of flexibility in label layout and design, provided the required elements are present (e.g., classification, wavelength, power, and hazard symbol). In contrast, the FDA mandates a specific label format with prescribed wording and layout. For CDRH labelling according to 21 CFR 1040.10 classification, product information, and the laser warning symbol labels must be combined into one label.
The label must include:
- The exact signal word “CAUTION” (for Class I and II lasers) or “DANGER” (for Class III and IV).
- A boxed label format with structured fields.
- A directive to avoid direct exposure to the beam.
The CDRH regulations use a non-ISO-formatted laser symbol and different safety sign formats.

APERTURE LABEL
21 CFR requires a non-removable aperture label at or near the laser beam exit point. Aperture labels must be located at all apertures that emit laser radiation. Aperture Labels are required on Class II, III and IV laser products.
Additionally, a separate output specification label is often required, detailing:
- Wavelength(s)
- Maximum output power
- Emission duration
PROTECTIVE HOUSING LABEL & INTERLOCKED HOUSING LABEL
CDRH regulations are stricter on label placement as they must be clearly visible during operation, maintenance, and service. IEC permits a more flexible approach, sometimes allowing labels on the protective housing or inside covers, depending on access conditions.
Products with service-access panels that bypass safety interlocks must carry additional cautionary labels under 21 CFR. The IEC addresses this through interlocked housing labels but does not require the same specificity in language and formatting.
Shop Laser Warning Labels
LASER COMPONENT LABEL
The IEC’s definition does not include laser products intended for use as components, which are defined as laser products in the FDA definition. The IEC acknowledges that certain components or repair parts are laser products. The FDA requires that component and repair (or replacement) laser products comply with FDA’s standards.
It is strongly recommended to include a Laser Component Label on the component.

Laser Notice 56 for Laser Manufacturers
Since the advent of Laser Notice 56, manufacturers have enjoyed a straightforward path to compliance with the FDA CDRH regulations for laser products. Laser Notice 56 allows products to be tested to IEC 60825-1 (or other equivalents such as BS EN 60825-1) instead of 21 CFR 1040.10 and 1040.11. This allows manufacturers to bypass the complicated differences in the labelling requirements of the two standards.
Using Laser Notice 56 means a product complies with 21 CFR 1040.10 except where IEC 60825-1 varies. Most European manufacturers are already required to test laser products to IEC 60825-1 and additional testing to 21 CFR standards is often another long and expensive process.
If a laser product is being imported under this declaration, the following text must be present on the laser product:
- “Complies with FDA performance standards for laser products except for conformance with IEC 60825-1 Ed. 3., as described in Laser Notice No. 56, dated May 8, 2019.”
- “Complies with 21 CFR 1040.10 and 1040.11 except for conformance with IEC 60825-1 Ed. 3., as described in Laser Notice No. 56, dated May 8, 2019.”

FDA Reporting and Submissions
It’s important to note that all laser products (lasers, or systems incorporating lasers) imported into the U.S.A. must be reported to the FDA, and labels must align with the submitted report. Registration of a laser product with the FDA is critical to successfully importing lasers into the U.S.A.
Before a laser product can be registered with the FDA, it must have been classified to a Laser Safety Standard. The FDA / CDRH will accept laser products that have been classified to IEC 60825, as well as those which have been classified to 21 CFR 1040.10 and 1040.11. It is relatively rare for manufacturers outside the U.S.A. to classify to 21 CFR 1040.10 and 1040.11. European manufacturers are more likely to only have products classified to IEC 60825 which will need to use Laser Notice 56.
Compliance with FDA/CDRH laser labelling requirements under 21 CFR is not interchangeable with IEC/EN 60825-1. The U.S.A. regulations demand precise formats, wording, and label placements that differ significantly from international standards. Manufacturers and importers must review and, if necessary, redesign their labelling to align with CDRH specifications to ensure lawful distribution in the American market. Ignoring these differences risks non-compliance, product recalls, or import rejection.
For more information on labelling requirements to IEC / EN 60825-1, see this article.

Lasermet offer consultancy, testing, and FDA/CDRH reporting services. Get in touch to learn more!
Disclaimer:
This article is intended for informational purposes only. While every effort has been made to ensure the accuracy of the content based on the IEC 60825-1 standard and related resources, we make no representations or warranties, express or implied, regarding the completeness, accuracy, or applicability of the information provided. This content does not constitute legal or regulatory advice. Readers are advised to consult the relevant standards documentation and seek professional guidance to ensure full compliance with applicable regulatory requirements. We disclaim any liability for decisions made or actions taken based on the information contained herein.