SeriousLED: Professional LED Safety Signs from Lasermet
Clear Signs. Technology that lasts. Lasermet is pleased to announce the launch of SeriousLED, a new range of professional LED…
All of our laser safety training is now available online Dismiss
Clear Signs. Technology that lasts. Lasermet is pleased to announce the launch of SeriousLED, a new range of professional LED…

Override by definition means to bypass, replace, or take control over an existing setting, rule, or action. In everyday life,…

When dealing with lasers in the workplace, safety is non-negotiable. Whether in healthcare, research, manufacturing, or aesthetics, laser systems introduce…

Laser products entering the U.S.A. market are subject to FDA specific labeling rules that diverge from IEC / EN standards….

Last November 17–20, 2025, Lasermet Europe GmbH exhibited at MEDICA 2025. Although Lasermet participates in trade shows all around the…

Lasermet proudly announces the launch of the ICS-TOUCH II, the next generation touchscreen interlock controller and the direct upgrade to…
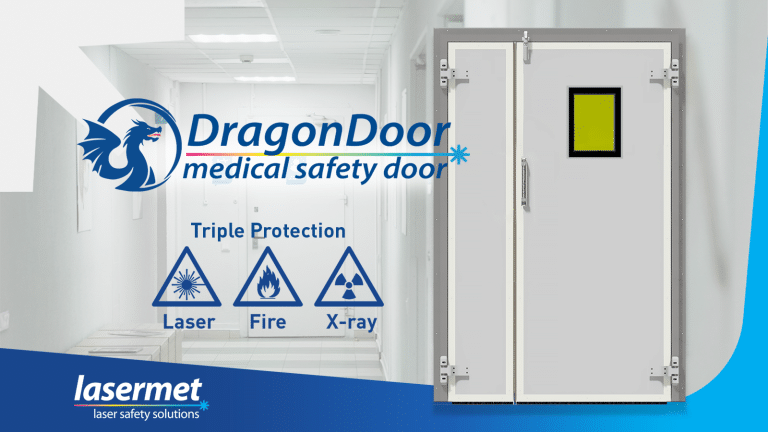
Lasermet is proud to announce the launch of the DragonDoor, the next evolution in safety for modern medical facilities. Purpose-built…

Lasermet held a successful and productive Distributor Conference at our Research and Development office in Cebu, Philippines. The event was attended by Lasermet directors Paul Tozer and Steve Geldard, along with distributors from across the globe.

Lasermet is proud to announce that we will be exhibiting at ICALEO 2025 (International Congress on Applications of Lasers &…

In industries where precision and safety are paramount—such as laser technology, medical devices, and electronics— the reliability of a test…